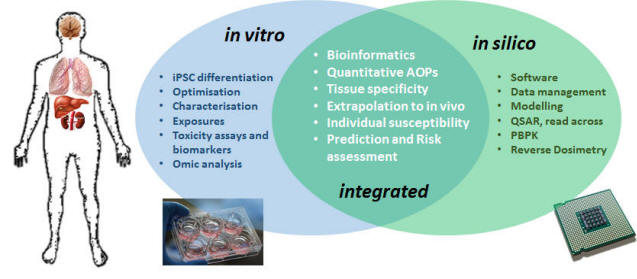
The in3 project is funded by the EU’s Marie Skłodowska-Curie Action – Innovative Training Network (MSCA-ITN for short) that aims to drive the synergistic development and utilisation of in vitro and in silico tools for human chemical and nanomaterial (NM) safety assessment. The project focuses on differentiation of human induced Pluripotent Stem Cells (hiPSC) to toxicologically relevant target tissues including; brain, lung, liver and kidney. The tissues, from the same genetic backgrounds, are exposed to common compounds and the data generated and prediction tools genereated will be used to develop moderinised safety assessment approaches combining cheminformatics, mechanistic toxicology and biokinetics into computational models which can account for donor and tissues specific effects. The project empolys 15 PhD students to carry out these activities in a coordinated and highly collaborative fashion.
Core scientific activities: Differentiation of well-characterised human iPSC into brain, lung, liver, kidney and vascular cellsDelineation of tissue specific and donor specific effects of compound exposures (uptake, metabolism, extrusion, and mechanistic toxicity)Development and optimisation of quantitative adverse outcome pathways (qAOPs) for each target organ which will be unified in an organism-level modelOptimisation of QSAR and read-across tools for safety assessmentUltimately to create a unified expandable integrated testing strategy for chemical and NM safety assessment
Project Background
Chemicals, including drugs and cosmetics, reaching the marketplace need to be tested for their potential to harm the environment and humans. In addition, manufactured nanomaterials (NMs) are also of concern. Safety evaluation is currently achieved for the most part utilising non-human whole animal tests. While animal models aim to predict a large amount of human biology they differ in significant ways in the hndling of many chemicals and pharmaceuticals, primarily due to species differences in phase I and II metabolizing enzymes and in chemical transporters. Additionally, there are ethical concerns pertaining to the use of animals for safety evaluation and thus the EU is actively supporting animal alternatives via the Reduction Refinement and Replacement (3Rs) of animal use in testing. Moreover, public pressure is still high and one of the three European citizenship initiatives that passed the one million signatures threshold in 2015, centred on banning animal testing in the EU. This momentum towards animal free chemical safety evaluation is outpacing the development of suitable in vitro and in silico replacement models and thus strategies that could be practically used across industry and regulatory organisations are urgently needed.
Induced Pluripotent Stem Cells: a revolution in biology
Over the past decade there has been a major focus on human cell lines and primary cells for tools in chemical safety assessment. Many cell lines however, suffer due to the fact that they exhibit cancerous phenotypes and thus have, by definition, an abnormal cell physiology. While primary cells recapitulate normal cell physiology to a greater extent, it is difficult to obtain a continuous source of high-quality material and therefore reproducible results. Furthermore, it is practically impossible to collect some tissues such as brain. In 2006, Takahashi and Yamanaka discovered a method to generate stem cells from somatic cells by overexpressing the transcription factors Oct3/4, Klf4, Sox2 and c-Myc in cultured fibroblasts. Astonishingly, the reprogrammed cells behaved like pluripotent stem cells, could self-renew indefinitely and could be differentiated into all three germ layers, i.e. the mesoderm, endoderm and ectoderm. The Nobel Prize in Physiology or Medicine 2012 was jointly awarded to John B. Gurdon and Shinya Yamanaka for this discovery underscoring the potential impact of this methodology. The methods to induce stemness have improved over the years using non-integrating techniques such as self-replicating RNA or Sendai virus. Also a lot of progress has been made in developing protocols to differentiate iPSC into specific cell types including neural, lung, liver, kidney and vasculature lineages. Thus normal non-cancerous cells can now be acquired easily from skin biopsies, blood or even urine and re-programmed into iPSC from any individual and expanded practically indefinitely. This has a huge advantage over embryonic stem cells, as the donors are living, they can be acquired from diseased individuals, there are less ethical issues and avenues such as patient-specific regenerative medicine and patient-specific drug design are now possible. The Innovative Medicines Initiative (IMI) has launched two large projects StemBANCC and EBiSC to create standards and iPSC biobanks as a resource of standardised, quality assured iPSC. Indeed, the StemBANCC project alone promises to generate and biobank iPSC from up to 500 individuals by 2017 and has achieved approximately half already. While many of these patients have specific disease related mutations there are also a large number of single nucleotide polymorphisms in drug-metabolising and drug-transporting genes.
Toxicology: a modern data rich discipline
Toxicity is a highly complex process critically dependent on a number of factors including chemical structure/state, tissue concentration, inherent biological activity-reactivity of the chemical or metabolite, cellular defence and repair mechanisms and the function of the target tissue. The modern day approach to toxicology is to focus on mechanisms, rather than the more traditional histopathological approach as understanding the mechanisms of toxicity allows better prediction and safer by design approaches. This mechanistic approach has benefited greatly from the use of transcriptomics and related methodologies which allow hypothesis-free, unbiased experimentation with extremely high-content data However, high-content data streams pose a problem for their application to risk assessment as specialist knowledge is often required. A potential solution is to create contextual frameworks of the evolution of a toxic event, from its molecular initiating event (MIE), to secondary, tertiary and downstream cascading key events (KEs), leading eventually to a pathology or adverse outcome. This concept of Adverse Outcome Pathways (AOP) is gaining momentum as a pragmatic method to enable the application of deeply mechanistic data to chemical risk assessment steered by the OECD and the EU regulatory agencies. The development of quantitative AOPs will require in vitro data, knowledge of disease progression and computer modelling approaches. in3 will build on previous knowledge (e.g. from the EU FP7 projects COSMOS, DETECTIVE, HeMiBio, ToxBank and Predict-IV), by focusing to mechanistic studies, linking exposure to hazard, creating biokinetic models and integrating in vitro data with in silico tools.
in3 aims to build on our collective experiences and achievements in projects such as Predict-IV, StemBANCC, eNanoMapper, NanoPUZZLES, the SEURAT1 clusters HeMiBio, COSMOS, DETECTIVE, ToxBank, and the newly launched Horizon 2020 project EU-ToxRisk. All of these projects focused to specific aspects of animal-free chemical and NM safety assessment. Here we will bring these innovations under a single training umbrella, focusing to mechanistic studies as a basis for hazard assessment, linking exposure to hazard, creating biokinetic models and integrating in vitro data with in silico tools.
 |
in3 is funded by the Marie Sklodowska-Curie Action – Innovative Training Network under grant no. 721975. |
 The ESTIV Members Area
The ESTIV Members Area